I attended the “Seminar for Microbiology Laboratories” organized by HKAS from 16 to 18 November 2010. The objective of the seminar is to discuss the current legislative requirement in the EU food and feed sectors particularly in relation to microbiological specifications and testing. In-depth discussion will be held in the seminar on method validation, estimation of measurement uncertainty, internal quality control plans, proficiency testing and other technical issues related to microbiological testing.
The speaker was Dr. Susan Passmore (BSc in Microbiology and PhD from
I would like to summarize the seminar for sharing purpose.
Session 1
Standard methods, legislation and laboratory accreditation
In this session, Dr. Susan Passmore introduced “Microbiological Specifications”, “Methods and Standards”, “Applicable Legislation” and “Laboratory Accreditation”.

There were several types of “Microbiological Specifications”, for instance, specification for raw materials, in-processing guidelines, end-product check, as well as, between manufacturer and retailer. Moreover, standards for official use included EU Regulation 2073/2005 – Microbiological criteria for foodstuffs (amended by 1441/2007 and 365/2010).
One of the common use specifications was Codex Alimentarius 2004 which concerned hygiene and safety. In order to fulfil the purposes of a microbiological specification, consideration should be given to:
· Evidence of hazards to health
· Microbiological status of raw materials
· Effect of processing on microbiological status of the food
· Likelihood and consequences of microbial contamination and / or growth during subsequent handling, storage (e.g. refrigerator may not be ideal) and use
· Category(s) of consumers at risk
Several standard methods were commonly used such as BSI, ISO, CEN (European Committee for Standardization), IDF (International Dairy Federation), AOAC, CODEX (analytical only), etc.
The following were the criteria for Methods of Analysis (EU OFFC REg 882/2004).
· Accuracy
· Applicability (matrix/concentration)
· Precision: repeatability intra-laboratory (within lab); reproducibility inter-laboratory (between labs)
· Limits of detection / determination
· Sensitivity* and Specificity (Selectivity)
· Linearity
· Measurement Uncertainty
· Other criteria that might be selected as required.
(* Sensitivity could mean “no false –ve”.)
The diagram showed the PT result summary.
Then Dr. Susan Passmore briefed the ISO 17025 Laboratory Accreditation based on European co-operation for Accreditation publication “EA-04/10 – Accreditation for Microbiological Laboratories”.
Session 2
Food and other legislation in the EU and
Dr. Susan Passmore said the purpose of Food Legislation used for protection of health and for promotion of fair trade. Different levels of regulatory were showed.
Then she introduced the European Union members included:
· Older Member States (France, Netherlands, Germany, Italy, Belgium, Luxembourg, Denmark, Ireland, UK, Austria, Finland, Greece, Portual, Spain and Sweden)
· Newer and Newest Member States (Cyprus, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Malta, Poland, Slovenia, Slovakia, Romania and Bulgaria)
EU Legislation included “Regulations”, “Decisions” and “Directives”. For Directives, it was separated into “Vertical” and “Horizontal”.
For Vertical: Fresh Meat, Minced Meat, Meat Products, Egg Products, Poultry Meat and Shellfish, etc.
For Horizontal: Hygiene of Foodstuffs, Official Control of Foodstuffs
The following diagrams demonstrated the step for adoption of a European Commission Proposal and the Consultation Procedure.
Before end of this session, Dr. Susan Passmore mentioned the following regulations for
For Food:
· Food Safety Act 1990 (Amendment) Regulations (2004)
· General Food Law Regulations 2004.
For Water:
· Water Industry Act (1991) (EU Water Directive 80/778/EEC)
· Natural Mineral Water Regulations (1985) (Natural Mineral Water Directives 80/777/EEC and 2009/54/EEC)
· Drinking Water in Containers Regulations (1994) (The Water Quality for Human Consumption Directive 98/83/EC)
For Cosmetics:
· Cosmetic Products Directive 76/768/EEC
· UK Cosmetics Products (Safety) Regulations (2008) – shelf-life declarations and permitted additives.
Session 3
Current EU food legislation – 2073 / 2005
In this session, Dr. Susan Passmore described the Food Hygiene Legislation – EU 2073/2005. It was a generic regulation on microbiological criteria for foodstuffs. The structure of 2073/2005 included three chapters.
· Chapter 1 – Food safety criteria
· Chapter 2 – Process hygiene criteria for meat, milk and dairy, egg, fishery, vegetables and fruits
· Chapter 3 – Sampling and sample preparation
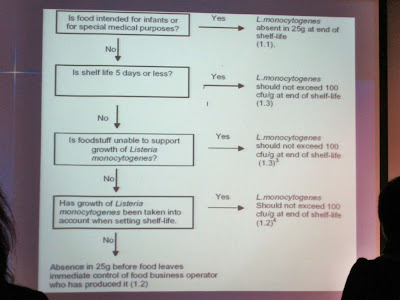
The following diagram showed the food categories having Listeria monocytogenes criteria.
Criteria for Enterobacteriaceae, E.coli, coagulase positive staphylococci, meat and meat carcasses were discussed.
Session 4
ISO methods for food microbiology
Dr. Susan Passmore introduced current food standards including Enumerations (Pour plate, Spread plate, Spiral plate, MPN and Membranes) and Detections (Cultural, IMS, PCR, ELISA).
For Enumerations:
(Pour plate)
· EN ISO 4833:2003, ISO 4832:2006, ISO 21528-2:2004, ISO 7937:2004, ISO 15214:1998, ISO 15213:2003
(Spread plate)
· EN ISO 6888-1:1999 +A1:2003, EN ISO 6888-2:1999, EN ISO 7932:2004, ISO 21517-1:2008, ISO 21517-2:2008, ISO TS 10272-2:2006, ISO 17410:2001
(MPN)
· EN ISO 21871:2006, EN ISO 6888-3:2003, ISO 21518-1:2004, ISO 4831:2006, ISO 7251:2005, ISO TS 16649-3:2005, EN ISO TS 10272-3:2010, ISO 16649-1:2001,
For Detections:
(Cultural)
· EN ISO 6579:2002, EN ISO 11290-1, EN ISO 10272-1:2006, ISO TS 21872-1:2007, ISO TS 21872-2:2007, EN ISO 21567:2004, EN ISO 10273:2003,
(IMS – Immunomagnetic Separation)
· EN ISO 16654:2001
(PCR – Polymerase Chain Reaction)
· EN ISO 22174:2005, EN ISO TS 20836:2005, EN ISO 20837:2006, EN ISO 20838:2006
(ELISA)
· No ISO standard for ELISA methods currently
After the first day sessions, Mr. W.W. Wong (Sr. Accreditation Officer, HKAS) led the Q&A session.
Reference:
Session 2
European law – http://eur-lex.europa.eu/en/index.htm
Food Standards Agency – http://www.food.gov.uk
European Food Safety Authority – http://www.efsa.eu.int
Session 3
GRAIL databased for imported food products – https://grail.foodapps.co.uk/grail/general/home













沒有留言:
發佈留言