2020年4月5日星期日
Collective Masks Anti-Epidemic Capability (集體口罩抗疫力)
2011年9月22日星期四
Modern Traditional Chinese Medicine (TCM) Clinic
Recently, I consult a Chinese Medicine Doctor in Science Park and find that The Nong's Chinese Medicine Clinic (農本方中醫診所) is a Modern Traditional Chinese Medicine (TCM) Clinic. They used a modern approach of concentrated Chinese Medicine granules, supported with a computerized program of their Management System.

From patient registration, diagnosis to prescription dispensation, all steps are computerized. The prescription dispensation is not using traditional form of herds but using concentrated individual Chinese Medicine granules. Each bottom of granules are identified by using bar code system first and then weighted a specific amount into a cup based on the electronic balance.

Then they put all different granules into a large bottom and mixing using the mixer. After that the mixed medicine granules are packed automatically.

All medicines dispensed are instantly soluble in hot water and require no decoction. The final product is shown as following photo.

It is totally different compared with Traditional Chinese Medicine Clinic which using original TCM herb. (e.g. The herd in the following book.)

High efficient, scientific and reduce time!
However, I am not sure that the effect between the mixed concentrated individual Chinese Medicine granules and extracted by mixed the original CM herbs are the same or not.
Reference:
The Nong's Chinese Medicine Clinic - http://www.nongs.com/clinics/index.htm
Easily Confused Chinese Medicines in Hong Kong (香港容易混淆中藥) - http://bublog.hkbu.edu.hk/bublog/html/blog.jsp?bid=17&pg=blogdetail&eid=173
HKJCICM > CM Database > Easily Confused Chinese Medicines in Hong Kong - http://www.hkjcicm.org/cm_database/confused/all_e.aspx?chapter=1
2010年8月17日星期二
Yakult Light (More Health Care)
(One gram of sugar, like that of any other carbohydrate, provides 4 calories in a person's daily diet.)
Moreover, one of company souvenirs was the book entitled “How the lactic acid bacteria in your daily diet may inhibit cancer?” I summarized part of the book into my blog named “Cancer Prevention and Intestinal Bacteria” (See reference).
My wife bought the Yakult Light with the Yakult Boy (Toys) during International Conference & Exhibition of the Modernization of Chinese Medicine & Health Products (國際現代化中醫藥及健康產品展覽會暨會議) (13 August 2010).

Visit to Hong Kong Yakult Co. Ltd. - http://qualityalchemist.blogspot.com/2008/11/visit-to-hong-kong-yakult-co-ltd.html
Cancer Prevention and Intestinal Bacteria (I)
http://qualityalchemist.blogspot.com/2009/01/cancer-prevention-and-intestinal.html
Cancer Prevention and Intestinal Bacteria (II)
http://qualityalchemist.blogspot.com/2009/01/cancer-prevention-and-intestinal_23.html
2010年1月30日星期六
Comparison between ISO/IEC 17025 accredited laboratory and GLP recognized laboratory
Scope:
GLP is developed for conducting non-clinical laboratory studies that support applications for research or marketing permits for products regulated by the FDA.
ISO 17025 applied to all laboratories where testing and/or calibration are performed and/or formed part of inspection and product certification. In principle, ISO 9001 is adopted in ISO 17025.
Similarities between both requirements:
i) Requirements on organization and personnel
ii) Requirements on equipment, sampling and validation
iii) Requirements on reporting / record and control of records
A schematic diagram showing the overlapping and specific requirements between an ISO/IEC 17025 accredited testing laboratory and an OECD GLP facility.
(Source: Engelhard T. et al., 2003)
The differences are shown in the following table.

ISO/IEC 17025 accredited laboratory has approximately 70% of the managerial and technical issues of the GLP directives covered, while 30% can be provided as an extension of the existing laboratory quality management system.
The simple checklists for preparing HOKLAS (Hong Kong Laboratory Accreditation Scheme) ISO 17025 accreditation and GMP audit are shown as follows:
Checklist for HOKLAS assessment:
1. Documents authenticating that the applicant laboratory is a legal entity or part of a legal entity
2. Quality manual & Operation procedure manual
3. Latest audit schedule
4. Summary of the findings of the latest quality and management system review
5. Test/calibration procedure manual(s)
6. Measurement uncertainty estimation
7. CV’s and copies of qualification documents for new nominees for signatory/operator approval
8. Laboratory floor plan
9. Laboratory organization charts, with key positions clearly identified
10. Sample test/calibration records
11. Sample test/calibration reports
12. Relevant proficiency test reports
13. Scope of accreditation to be assessed
14. Other documents (please specify)
Checklist for GMP audit (included GLP):
1. Business Registration Certificate
2. Floor plan
3. Organization chart, personnel qualification and signature record
4. Information sheet of key personnel (Authorized Person, QC manager and Production manager)
5. No. of full time and part time staff for manufacturing, QC and packaging
6. Full product list (with breakdowns of dosage forms & formulations)
i. Type of sterile pharmaceutical dosage (such as eye drops, single-dose injections, etc.)
ii. Other type of pharmaceutical dosage (such as tablets, capsules, powders, etc.)
7. List of major production equipment and QC equipment
8. Validation schedule of manufacturing processes and test methods
9. Stability study schedule
10. Others
Reference:
21CFR58: GLP for Non-clinical Laboratory Studies
ISO/IEC 17025: General requirement for the competence of testing and calibration laboratory
Engelhard T., Feller E. & Nizri Z. (2003) “A comparison of the complimentary and different issues in ISO/IEC 17025 and OECD GLD” Accred Qual Assur, Vol. 8, pp208-212.
2009年3月22日星期日
Drug Recall and Related Testing Scope in Hong Kong Laboratories
For controlling of Chinese medicines, the Chinese Medicines Section of the Government Laboratory was established in 1998, as well as the relevant analytic methods, to examine Chinese medicine for possible contaminants and adulteration of western medicine. In order to support the enforcement of the Chinese Medicine Ordinance and to demonstrate the competence in laboratories, the accreditation scheme of ISO/IEC17025 was also developed.
The Government Laboratory is the first laboratory accredited under HOKLAS for TCM in mid-2002, when the analysis of toxic elements in Chinese medicine namely, arsenic, cadmium, mercury and lead adopted microwave digestion followed by ICP-MS measurement. It aims to stimulate other local laboratories performing similar tests to seek accreditation thereby upgrading their testing standard. After that several commercial laboratories have accredited different testing scope in Chinese Medicine category.

The directory of accreditation is posted in HKAS website at http://www.itc.gov.hk/en/quality/hkas/hoklas/directory/chin.htm , where
1 – Government Laboratory
3 – The Hong Kong Standards and Testing Centre Ltd.
4 – CMA Industrial Development Foundation Limited
5 – Intertek Testing Services Hong Kong Ltd.
9 – SGS Hong Kong Limited
39 – Hong Kong Productivity Council – Environment and Product Innovation Laboratory
58 – Bureau Veritas Hong Kong Limited - Kowloon Bay Office
66 – ALS Technichem (HK) Pty Limited
83 – Wellab Limited
145 – Institute for the Advancement of Chinese Medicine (IACM) Ltd.
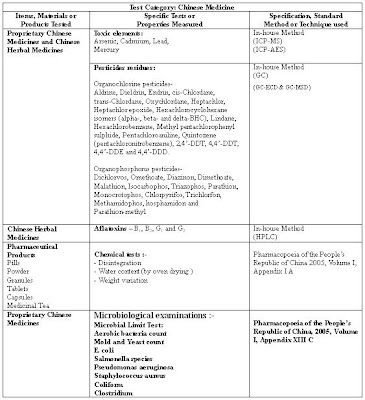
The summary of test scope for Chinese Medicine in Hong Kong Laboratories is shown in the following table.
2009年3月19日星期四
Differences between ISO 9001, HKGMP and ISO/IEC 17025
The fundamental differences in the processes used by accreditation bodies and certification bodies to establish compliance with ISO/IEC17025 (HOKLAS Accreditation) and ISO9001 are obviously. Laboratory accreditation aims to recognize specific technical competence, the assessments of laboratories are conducted by teams comprising relevant technical experts and assessors able to evaluate not only the compliance with the management systems requirements of ISO/IEC17025 (or ISO9001), but also determining the specific technical competence of personnel and the availability of all the technical resources need to produce reliable data and results for specific test methods(5).

Reference:
1. Lai, Lotto K.H. and H.Y. Cheung (2003) “Laboratory accreditation has added-value for quality control of herbal medicine manufacturing but cannot replace implementation of good manufacturing practices.” Hong Kong Pharmaceutical Journal, 12(1), pp26-19.
2. Cheung H.Y. and L.W. Chan (1998), Planning and Designing a GMP Plant for the Manufacture of Chinese Herbal Pills, International Symposium on “The Worldwide Herbal Industry: Present and Future”, p16, July 15-17.
3. Chin K.S., K.V. Patri, K.F. Pun, W.H. Yeung, L.T. Poon and K.K. Poon (1996), Starting the Journey: Team-based QI in a University Laboratory, The TQM Magazine, 8(2), pp.20-25.
4. Lau Edward P. (1996), Pharmaceutical Quality Assurance and Quality Control Workshop, E.L. Associates, Inc., USA.
5. HKAS News, Hong Kong Accreditation Service, Issue No.33, January 2003.
2009年1月24日星期六
Cancer Prevention and Intestinal Bacteria (II)
Most of the bacteria in the intestine can be divided into two categories: lactic acid bacteria (feed on sugars/carbohydrates) and putrefactive bacteria (feed on proteins).
One theory holds that these intestinal bacteria transform fat into a carcinogen. Another possibility is that – since a person eating meat or egg takes in protein as well as fat – the proteins are being transformed into carcinogens.
Putrefactive bacteria (e.g. Bacteroides, E. coli, Veillonella, and Clostridium) break down proteins into ammonia, hydrogen sulfide, amines, phenol, and indole. Phenol and indole are known promoters of intestinal and other cancers. (They are also components of coal tar.)
A portion of the phenol is reabsorbed by the digestive tract and is detoxified in the liver, either by combining with glucuronic acid or sulfuric acid to form a harmless compound which is then eliminated in the urine; or by being released into the intestines and mixed with bile. The glucuronic acid compound with the bile is reconverted to phenol by the action of an enzyme known as beta-glucuronidase (It is most active in putrefactive bacteria such as E. coli and Clostridium welchii.), which is produced by some intestinal bacteria.
 enterohepatic circulation diagram
enterohepatic circulation diagramLactic acid bacteria feed on sugars to produce lactic acid. Intestine dominated by lactic acid bacteria leans toward acidity that suppresses growth in putrefactive bacteria (which favor an alkaline environment). By weakening the influence of the putrefactive bacteria that produce carcinogens and induce enterohepatic circulation, Lactobacilli can play a significant role in improving the intestinal environment.
(The stool’s color is mainly from bilirubin, a reddish-yellow bile pigment produced in the liver from the hemoglobin contained in old red blood cells. Bilirubin’s color varies with acidity.
Acidic environment: Yellow
Neutral environment: Orange to brown
Alkaline environment: Greenish- or Blackish-brown.)
2009年1月19日星期一
Cancer Prevention and Intestinal Bacteria (I)
How cancer grows?
Cancer is a mass of tumor cells and each cancer cell is a normal cell that has undergone a transformation.
Growth factors and growth inhibitory factors are proteins that message to multiply, and the latter to stop multiplying, so as to main the certain number of cell.
The so-called accelerator is known as the proto-oncogene that controls the functions of receiving messages at the cell membrane. The gene responsible for applying the brakes to the cellular proliferation process is the tumor suppressor gene.
How genetic malfunction produces cancer cells?
Gene in a normal cell (proto-oncogene):
1. “Antenna” intercepts extracellular message.
2. Messages are correctly transmitted to the cell’s interior.
3. After being scrutinized and processed, messages are transmitted to nucleus.
4. Another gene within the nucleus is activated and initiates and cellular proliferation process.
Malfunctioning gene (oncogene):
1. Malfunctioning “antenna” does not receive messages, but continues to signal cells to multiply.
2. “Antenna” transmits messages not received from outside.
3. Erroneous messages described in (1) and (2) are passed on to the nucleus.
4. The cellular proliferation switch remains perpetually in the “on” position.

The next stage, extending from when a cancer cell develops until it begins proliferating, is considered a separate process. The process called promotion, and anything that instigates the process is known as promoter. (e.g. sex hormones, secondary bile acids, and chemicals such as saccharin, and artificial sweetener.)

Experiments have shown that vitamins C and E, as well as beta-carotent (a precursor of vitamin A) weaken the action of initiators, and that beta-carotent and vitamin A combat promoters within a cell.
One cancer cell does not constitute cancer
The smallest tumor detectable by various modern medical equipments consists of about a billion cells, weighs about 1g, and measures about 1 cm in diameter. (A cell weighs one one-billionth of a gram, even a growth of a million cancer cells weighs only about 1mg and is about 1mm in diameter.)
A stressful lifestyle or other factors can reduce the effectiveness of the cellular surveillance, rendering the body more likely to allow cancer to progress.
2008年5月7日星期三
Biotech Lab General Requirement
For Microbiology Laboratory, the facilities need to consider Biosafety Levels. WHO identified Risk Groups into 1, 2, 3 and 4. The table 1 is a classification of infective microorganisms by risk group.
 Table 2 is relation of risk groups to biosafety levels, practices and equipment
Table 2 is relation of risk groups to biosafety levels, practices and equipment1. The international biohazard warning symbol and sign (Figure 1) must be displayed on the doors of the rooms where microorganisms of Risk Group 2 or higher risk groups are handled.
2. Only authorized persons should be allowed to enter the laboratory working areas.
3. Laboratory doors should be kept closed.
4. Children should not be authorized or allowed to enter laboratory working areas.
5. Access to animal houses should be specially authorized.
6. No animals should be admitted other than those involved in the work of the laboratory.
Fig 1. Biohazard warning sign for laboratory doors

Laboratory design and facilities for BSL 1 & 2.
1. Formation of aerosols
2. Work with large volumes and/or high concentrations of microorganisms
3. Overcrowding and too much equipment
4. Infestation with rodents and arthropods
5. Unauthorized entrance
6. Workflow: use of specific samples and reagents.
Examples of laboratory designs for Biosafety Levels 1 and 2 are shown in Figures 2 and 3, respectively.
Fig. 2 A typical Biosafety Level 1 laboratory

The containment laboratory – Biosafety Level 3 is designed and provided for work
with Risk Group 3 microorganisms and with large volumes or high concentrations of
Risk Group 2 microorganisms that pose an increased risk of aerosol spread.
Fig. 4 A typical Biosafety Level 3 laboratory
 The maximum containment laboratory – Biosafety Level 4 is designed for work withRisk Group 4 microorganisms. (It is not allowed in Hong Kong.)
The maximum containment laboratory – Biosafety Level 4 is designed for work withRisk Group 4 microorganisms. (It is not allowed in Hong Kong.)For Molecular Biology Laboratory, the requirement to set up a PCR Laboratory is considered.
Development of the polymerase chain reaction (PCR) as a basic component of the molecular biology laboratory. The PCR laboratory typically is involved with activities that include sample preparation, PCR reaction assembly, PCR execution, and post-PCR analysis. Contamination prevention approaches are used in the PCR laboratory.
Fig.5 Outline of sample processing and analysis in a PCR laboratory.

Air handling:
In the pre-PCR laboratory, there should be a slight positive pressure compared to the air in the connecting hallway. The post-PCR laboratory, in contrast, should be at slightly reduced pressure to pull air in from the outside and thereby prevent escape of amplicons from the completed PCR samples being analyzed inside the lab
UV irradiation:
It uses UV to sterilize the entire pre-PCR laboratory.
Protective clothing:
To further prevent PCR amplicons from leaving the post-PCR lab, each investigator should have a dedicated post-PCR lab coat. Additionally, each investigator should have a general molecular biology lab coat and a separate coat for pre-PCR.
Adhesive paper at lab entrances:
This approach effectively prevents trace amounts of dust and debris from entering the laboratory.
Reference:
1. Laboratory biosafety manual (Third edition) 2004: By World Health Organization
2. Setting Up a PCR Laboratory: By Theodore E. Mifflin (Department of Pathology, University of Virginia, Charlottesville, Virginia 22908)