The speaker was Mr. Daniel Tholen (Technical Consultant, The American Association for Laboratory Accreditation (A2LA)) and his presentation content included the follows:
1. ISO Guides for Reference Materials
2. Guide 34 requirements in Guide 35
3. Guide 34 requirements not in Guide 35
In the beginning, Mr. Daniel Tholen briefed different ISO guides for reference material and its revisions.
- Guide 30: Terms and Definitions for Reference Materials
- Guide 31: Contents of Certificates and Labels
- Guide 34: General Requirements for Competence of Reference Material Producers
- Guide 35: General and Statistical Principles for Certification (Tentative revision title: General Guidance for the Assignment of Property Values)
Other new guidance documents were also mentioned such as:
- Internal Production of RMs for QC (Guide 80)
- RMs for Nominal properties (TR 79)
- Guidance for use of RMs (Guide 33)
The discussion would be expected on next REMCO meeting in July, USA.
"Guide 34 requirements in Guide 35" included Quality Policy (4.1.2) and follows:
- 5.12 Metrological traceability
- 5.13 Assessment of homogeneity
- 5.14 Assessment of stability
- 5.15 Characterization
- 5.16 Assignment of property values and their uncertainties
However, the current approach in Guide 35 did not design for situation of commercial Reference Material Producers (RMPs) because of Cost Considerations, Time to Market and Uncertainty. The guide was also difficult for many accreditation bodies use because of Statistics, Traceability and Uncertainty.
"Guide 34 requirements not in Guide 35" were shown as follows:
- Non-certified RMs (e.g. QC and PT)
- Demonstrating equivalence of replacement batches
- Uncertainty in the presence of degradation
- Qualitative RMs
- Stability under usage conditions (might not be addressed in revision)
The confusing topics in Guide 35 were discussed as follows.
- Minimum uncertainty for homogeneity must be > 0
- Minimum uncertainty for stability must be > 0
- Short term stability vs. Transportation
- Characterization in one laboratory using a single (primary) method
The proposed changes from latest circulation drafts were showed in the following slides.
Homogeneity testing was usually necessary and was able to use estimates of uncertainty from previous batches of similar material. (where Ubb - Uncertainty between bottle; Sr - repeatability)
The following criteria used for determining minimum number of units to test for homogeneity. (where Nprod - No. of production)
Other consideration of Homogeneity included to check:
- Within-unit homogeneity
- Trend in testing order and in production order
- Outlier difference between replicates
- the use interlaboratory nested design
- report minimum sample amount
Stability general consideration included use of information from previous batches of similar material, and allow u(lts) = 0, and it should test all properties. (where u(lts) - uncertainty of long term stability)
Short term Stability:
- Transportation
- Storage by user under alternative conditions
Long term Stability:
- Classical, or real-time studies
- Isochronus studies
- Accelerated studies
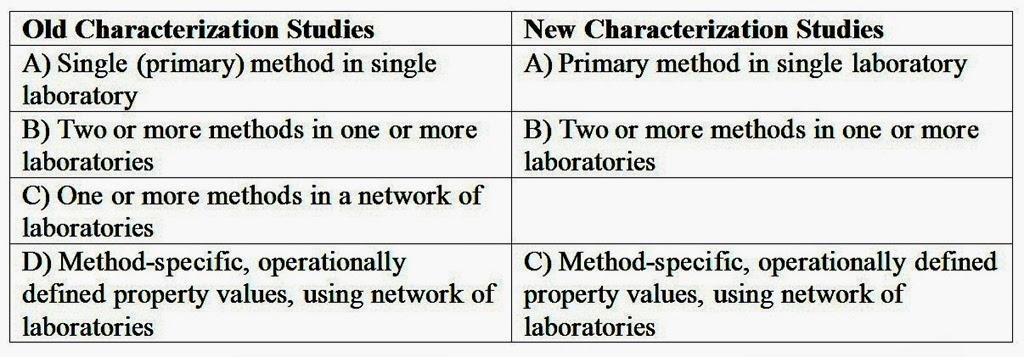
Characterization four types of studies as discussed in G34 was proposed to combine into three types of studies as follows.
Special issues of characterization on CRMs such as Purity, Secondary, Identity, Presence/Absence, Ordinal & Qualitative Properties, as well as Uncertainty were discussed.
Reference:
HKAS - http://www.itc.gov.hk/en/quality/hkas/about.htm
A2LA Seminar Leaders - http://www.a2la.org/training/biographies.cfm
Homogeneity testing was usually necessary and was able to use estimates of uncertainty from previous batches of similar material. (where Ubb - Uncertainty between bottle; Sr - repeatability)
The following criteria used for determining minimum number of units to test for homogeneity. (where Nprod - No. of production)
Other consideration of Homogeneity included to check:
- Within-unit homogeneity
- Trend in testing order and in production order
- Outlier difference between replicates
- the use interlaboratory nested design
- report minimum sample amount
Stability general consideration included use of information from previous batches of similar material, and allow u(lts) = 0, and it should test all properties. (where u(lts) - uncertainty of long term stability)
Short term Stability:
- Transportation
- Storage by user under alternative conditions
Long term Stability:
- Classical, or real-time studies
- Isochronus studies
- Accelerated studies
Characterization four types of studies as discussed in G34 was proposed to combine into three types of studies as follows.
Special issues of characterization on CRMs such as Purity, Secondary, Identity, Presence/Absence, Ordinal & Qualitative Properties, as well as Uncertainty were discussed.
Reference:
HKAS - http://www.itc.gov.hk/en/quality/hkas/about.htm
A2LA Seminar Leaders - http://www.a2la.org/training/biographies.cfm





沒有留言:
發佈留言