
Mr Bryan MK SO (Hon Secretary, Biomedical Division of HKIE) introduced the speaker Mr. Matthew Kopecky (Director of Sales Support, Asia Pacific of Sparta Systems, Inc.).

The following slide showed the parallels in Quality Control and Quality Assurance.Quality Management Solution enables organizations to define, track and manage the data and process associated with business events and maintains seamless compliance.

The End to Eng Quality Management was mentioned.

Mr. Kopecky introduced the cores of quality management system (QMS) below:
- Event Management & CAPA (Corrective Action and Preventive Action),
- Complaints,
- Audits (internal & external), and
- Change Control.
For complaint, all events must be documented, assessed and potentially investigated.
There were three common complaint sources (or types) below.
1. Call Centers
2. Customer Inquiries
3. Field Service
The fishbone diagram for CAPA was shown as follows.

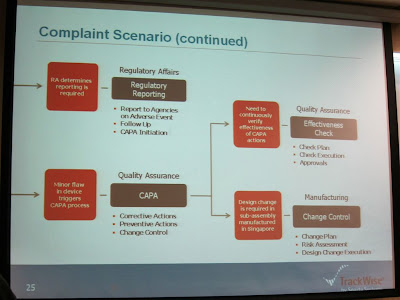
The complaint scenario flow was demonstrated.

Mr. Kopecky described different type of audit:
- Corporate Compliance Audits
- Internal Department (QA) audits
- Issue-specific Audit (e.g. specific in problem area)
- Audit to Confirm Effectiveness of Action (Follow-up audit)
- Supplier Quality Audit
- Financial Audit
- Computer Systems Validation (CSV) Related audit
- Third Party Audit (e.g. FDA, ISO, etc.)

One of the important core elements in QMS was “Change Control”. Because the process of requesting, implementing and documenting any change that might impact the quality of the product. Those change included Batch, Material, System, Supplier, Equipment, IT and Document.

And then he introduced an electronic quality management solution (EQMS) included selection criteria, deployment, integration, validation and training. It was because of the need of current day business trends (System requirements):
- Global Operations (Multiple sites & Languages)
- Expanding manufacturing and supply chains (Web-based and Scalable)
- Regulatory Pressures (Compliance with cGXP and meet regional & local regulations)
- Enterprise systems (Ability to integrate with other systems such as ERP, LIMS, CRM, etc.)
Six stage deployment methods were briefed and they were Analyze Requirements, Design & Build, Test, Validate, Train, and Go Live (or Support).
The integration of common ERP was provided through Integrated Enterprise Solutions.

Lastly, Mr. Kopecky shared case about Medical Device Product Complaint Management to us. He demonstrated EQMS fulfilled the following product requirements:
- Off-the-shelf, no or limited customization;
- Easily configurable such as user friendly, intuitive and minimum training;
- Compliance with auditing standards such as ISO, COSO rules, etc.; andCompatibility with EMEA’s IT platform.








































