Lecture 1 (16 April 2010):
Classification of Poisons
Labelling of Poisons and Pharmaceutical Products

The summary of Lecture 1 were shown as follows.
Laws concerned the following ordinances.
- Pharmacy and Poisons Ordinance (Cap. 138) – PPO
- Antibiotics Ordinance (Cap. 137) – AO
- Dangerous Drugs Ordinance (Cap. 134) – DDO
- Undesirable Medical Advertisements Ordinance (Cap. 231) – UMAO
- Chinese Medicine Ordinance (Cap. 549) – CMO
- Import and Export Ordinance (Cap. 60) – IEO
- Control of Chemicals Ordinance (Cap. 145) – CCO
- Public Health and Municipal Services Ordinance (Cap. 132) – PHMSO
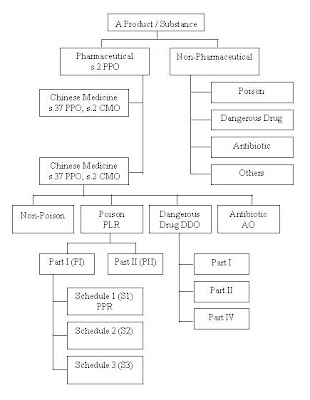
The chart showed the classification of Drugs
 Then labeling of poisons and pharmaceutical products in Cap. 138 were introduced.
Then labeling of poisons and pharmaceutical products in Cap. 138 were introduced.General labeling requirements for registration of pharmaceutical products included the following items.
1. Name of the product
2. Name and quantity of each active ingredient
3. Name and address of the manufacturer
4. Hong Kong registration number of the product
5. Batch Number
6. Expiry date
7. Specific storage conditions, if any
For sterile products, additional labeling requirement were need.
1. Name of preservative, if any
2. batch number and expiry date
For more information:
www.legislation.gov.hk
http://www.pshk.hk/
沒有留言:
發佈留言