I attended the seminar entitled "The Meaning of Accreditation to Medical Testing" which organized by Hong Kong Council for Testing and Certification (HKCTC) and Hong Kong Accreditation Service (HKAS) on 31 January 2011. I would like to summarize the seminar below for sharing.

Mrs. Marianne Leung (Member of HKCTC) gave an opening remarks. She said accreditation to medical testing was familiar in the west. Eventhough we had doing a good thing but the accreditation could open our mind and improvement our standard continuously.

The first speaker was Ms. Bella Ho (Senior Accreditation Officer, HKAS) and her topic was "Accreditation Service for the Medical Laboratories".

Ms. Ho introduced the background of HKAS. There are three scheme under HKAS included HKCAS, HOKLAS and HKIAS. Then she briefed the international recognition through mutual recognition arrangement (MRA) of ILAC and APLAC, as well as, multilateral recognition arrangement (MLA) of IAF and PAC.

Then she mentioned the structure of HKAS. The following diagram showed the details. She said about 100 assessors for ISO 15189 and around 20 assessors for each field. Ms. Ho questioned us what is accreditation. Then she used a simple terms to explain it as "Accreditation is a formal recognition that an organization is competent to carry out specific task to an acceptable standard".

The history of medical programme for ISO 15189 in Hong Kong was reviewed.

Ms. Ho introduced the accreditation process flow for applying HOKLAS accreditation.

Assessment team constitution included HKAS officer, one pathologist and one scientist for each discipline.

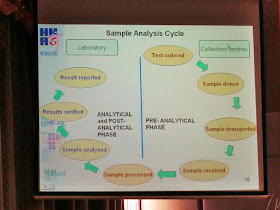
The whole sample analysis cycle included pre-analytical, analytical and post-analytical phase were briefed. All steps should be reviewed during assessment.
Until January 2011, 31 accreditations granted to 86 laboratories. All 13 laboratories were under Department of Health; and the other 52 laboratories were in 13 Hospital under HA. The remaining 21 laboratories were in private sector including 46 collection centres operated by these laboratories and 5 laboratories in 4 private hospitals. The details showed in the following diagram.

The scope of ISO 15189 accreditation in HOKLAS included Anatomical pathology, Chemical pathology, Immunology, Haematology, Microbiology, Molecular genetics and Cytogenetics.

Finally, Ms. Ho concluded that HOKLAS means the commitment to Quality.

The second speaker was Dr. Michael Suen (President of Hong Kong College of Pathologists) and his topic entitled "Laboratory Accreditation - How Useful Is It?"

Dr. Suen briefed the laboratory practice which was separated into Manual Work, Equipment and Automation.

Then he mentioned the quality control practice.

After that he explained how necessary/important of responsibility, Accountability and Liability.
For Responsibility, it was related to the role of Medical Laboratory and Reference Laboratory.
For Accountability, people would ask 'Who', 'What', 'How', 'Why' and 'When', if accident / error appeared.
For Liability, it was a responsiblity according to law (Action leading to Result - Medical Legal).

Dr. Suen shared one of accident investigation case to us. He said that the accreditation of laboratory was very important to show the integrity of result and traceability of the work. The safeguard of quality through accreditation included Document Control, QA Program, Delegation of Duties, Quality Meeting and Safety.

Lastly, Dr. Suen concluded that the accreditation was useful because of the followings: Record Tracing, Push for Quality (Not Guarantee for Quality), Social Consciousness, Management Incentive and Liability Issue.

After tea break, Experience Sharing from Two Accredited Medical Laboratories: Road to Accreditation was started.
Ms. Tam Chung Mei (Laboratory Manager, PathLab Medical Laboratories Ltd.) shared her laboratory accreditation journey.

Firstly, she introduced the background of PathLab Medical Laboratories Ltd.

Then she explained why choose HOKLAS. It was because of Long term cost savings and mutual recognition.

Ms. Tam shared their experience within 2-year preparation time. Getting started the process, a key responsible person was designated. They met problems with old documents. After completed the document control, they needed to prepare technical procedures and processes. Internal Quality Control should be implemented and Proficiency Testing - External Quality Assurance Programs (EQAP) should be participated. The most costly was method validation! Calibration and verification of different equipment (such as Tachometer, Balance, Centrifuge, Pipette, Thermometers, Timer and Autoclave) were must. She assigned two auditors that one was for management system and the other was for technical requirement. Lastly, management and staff meetings were implemented.

Ms. Tam concluded four benefits for accreditation as 'Improved overall efficiency', 'Standardization', 'Improved staff morale' and 'Recognition'.

The last speaker was Mr. Tsang Chun Ho (Laboratory Director, Onco Medical Laboratory Ltd.) and he shared his view on accreditation.

Mr. Tsang briefed his key concept for accreditation was continuous improvement and business sustainability. The most important was Positive Thinking!

Then he introduced Onco Med Lab Ltd services included Pap Smear and Blood Tests.
He said that the most important factors for accreditation was Standard Operation Procedure (SOP) and Staffing.

Mr. Tsang shared the benefits after accreditation below:
l Reduce number of customer complaints, incidents and accidents, as well as error result and number of missing specimens.
l Good Image, Good QC
l Good record system (Traceability)
l Technical development
l Doctors attitude overall were positive to laboratory accreditation

At the end, Mr. Tsang used computer to simulate the relationship of Soft Culture and Accreditation to Continuously Improvement. In additional, he use a famous Chinese sentence to end the talk.
(錢不是萬能, 但沒錢是萬萬不能.) Similar to
(Accreditation不是萬能, 但沒Accreditation是萬萬不能.)
Q&A Session:
There were some questions raised from participants for discussion such as backdated record signature, auditing, staff morale etc.

Reference:
Other related information:
Quality Management for Laboratory Training by HKSQ & CityU: http://www.hksq.org/HKSQ_BCH_2010_11_18_R4.pdf
Certified Laboratory Quality Specialist CLabQS (HKSQ) Registration: http://www.hksq.org/cert_hksq_clabqs.htm
History of Certificate Course on Quality Management for Laboratory:
QA:
回覆刪除祝免年旺相,事事吉祥!