For controlling of Chinese medicines, the Chinese Medicines Section of the Government Laboratory was established in 1998, as well as the relevant analytic methods, to examine Chinese medicine for possible contaminants and adulteration of western medicine. In order to support the enforcement of the Chinese Medicine Ordinance and to demonstrate the competence in laboratories, the accreditation scheme of ISO/IEC17025 was also developed.
The Government Laboratory is the first laboratory accredited under HOKLAS for TCM in mid-2002, when the analysis of toxic elements in Chinese medicine namely, arsenic, cadmium, mercury and lead adopted microwave digestion followed by ICP-MS measurement. It aims to stimulate other local laboratories performing similar tests to seek accreditation thereby upgrading their testing standard. After that several commercial laboratories have accredited different testing scope in Chinese Medicine category.

The directory of accreditation is posted in HKAS website at http://www.itc.gov.hk/en/quality/hkas/hoklas/directory/chin.htm , where
1 – Government Laboratory
3 – The Hong Kong Standards and Testing Centre Ltd.
4 – CMA Industrial Development Foundation Limited
5 – Intertek Testing Services Hong Kong Ltd.
9 – SGS Hong Kong Limited
39 – Hong Kong Productivity Council – Environment and Product Innovation Laboratory
58 – Bureau Veritas Hong Kong Limited - Kowloon Bay Office
66 – ALS Technichem (HK) Pty Limited
83 – Wellab Limited
145 – Institute for the Advancement of Chinese Medicine (IACM) Ltd.
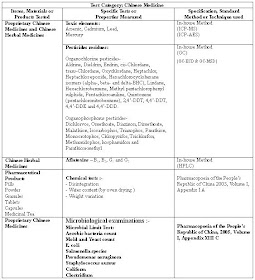
The summary of test scope for Chinese Medicine in Hong Kong Laboratories is shown in the following table.
Recently, the Department of Health (DH) invites Professor Yuen from Dept. of Microbiology in Hong Kong University to perform a feasibility study about the introduction of microbiology tests in pharmaceutical manufacturing industry.
沒有留言:
發佈留言