Scope:
GLP is developed for conducting non-clinical laboratory studies that support applications for research or marketing permits for products regulated by the FDA.
ISO 17025 applied to all laboratories where testing and/or calibration are performed and/or formed part of inspection and product certification. In principle, ISO 9001 is adopted in ISO 17025.
Similarities between both requirements:
i) Requirements on organization and personnel
ii) Requirements on equipment, sampling and validation
iii) Requirements on reporting / record and control of records
A schematic diagram showing the overlapping and specific requirements between an ISO/IEC 17025 accredited testing laboratory and an OECD GLP facility.
(Source: Engelhard T. et al., 2003)
The differences are shown in the following table.
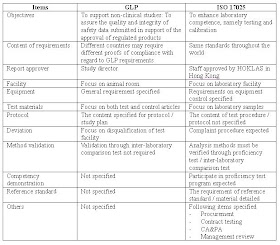
ISO/IEC 17025 accredited laboratory has approximately 70% of the managerial and technical issues of the GLP directives covered, while 30% can be provided as an extension of the existing laboratory quality management system.
The simple checklists for preparing HOKLAS (Hong Kong Laboratory Accreditation Scheme) ISO 17025 accreditation and GMP audit are shown as follows:
Checklist for HOKLAS assessment:
1. Documents authenticating that the applicant laboratory is a legal entity or part of a legal entity
2. Quality manual & Operation procedure manual
3. Latest audit schedule
4. Summary of the findings of the latest quality and management system review
5. Test/calibration procedure manual(s)
6. Measurement uncertainty estimation
7. CV’s and copies of qualification documents for new nominees for signatory/operator approval
8. Laboratory floor plan
9. Laboratory organization charts, with key positions clearly identified
10. Sample test/calibration records
11. Sample test/calibration reports
12. Relevant proficiency test reports
13. Scope of accreditation to be assessed
14. Other documents (please specify)
Checklist for GMP audit (included GLP):
1. Business Registration Certificate
2. Floor plan
3. Organization chart, personnel qualification and signature record
4. Information sheet of key personnel (Authorized Person, QC manager and Production manager)
5. No. of full time and part time staff for manufacturing, QC and packaging
6. Full product list (with breakdowns of dosage forms & formulations)
i. Type of sterile pharmaceutical dosage (such as eye drops, single-dose injections, etc.)
ii. Other type of pharmaceutical dosage (such as tablets, capsules, powders, etc.)
7. List of major production equipment and QC equipment
8. Validation schedule of manufacturing processes and test methods
9. Stability study schedule
10. Others
Reference:
21CFR58: GLP for Non-clinical Laboratory Studies
ISO/IEC 17025: General requirement for the competence of testing and calibration laboratory
Engelhard T., Feller E. & Nizri Z. (2003) “A comparison of the complimentary and different issues in ISO/IEC 17025 and OECD GLD” Accred Qual Assur, Vol. 8, pp208-212.



.JPG)













