In day 2, Dr Susan Passmore continued her lectures as follows.
Session 5
General ISO microbiology standards and water methods
Firstly, Dr. Susan Passmore introduced the general food standards such as EN ISO 7218:2007 – General requirements and guidance for microbiological examinations and EN ISO 6887 series – preparation of test samples, initial suspension and decimal dilutions for microbiological examination; as well as, EN ISO 16140:2003 – Protocol for the validation of alternative methods.

ISO 21807:2004 – Determination of water activity and ISO TS 19036:2006+A1:2009 – Guidelines for the estimation of measurement uncertainty for quantitative determinations were also discussed.
Other standards were also briefed.
(Milk)
· ISO 14461-1:2005, ISO 14461-2:2005
(Water Quality)
· ISO 8199:2007, ISO 19458:2006, EN ISO 17994:2004, ENV ISO TR 13843:2001, EN ISO 9308-1:2000, ISO 9308-2:1990, ISO 9308-3:1999, EN ISO 7899-1:1999, BS EN ISO 7899-2:2000, etc.
She also mentioned the cosmetics legislation such as Directive 76/768/EEC and UK Consumer Products (Safety) Regulations 2008 SI 1284/2008; and cosmetics standards (See table below).
Session 6
Method validation and laboratory competence
Dr. Susan Passmore stated the ISO 17025 requirements which related to method validation as follows:
·
·
·
Some standards for validation of microbiological were mentioned such as ISO 16140:2003, ISO TR 13843:2001, ISO 17994:2004, ISO 7218:2007, EA-2/06, EA-4/10 (section 4) and IUPAC Harmonized Guideline for Single Laboratory Validation of Methods of Analysis.
The development of ISO 16140 (Microbiology of food and animal feeding stuffs -- Protocol for the validation of alternative methods) included six project group (PG) below.
· PG1 – Terminology
· PG2 – Validation of proprietary methods
· PG3 – Intermediate validation
· PG4 – Method verification
· PG5 – In-house validation
· PG6 – Requirements for elaboration or revision of standard methods
Then Dr. Susan Passmore discussed the elements of laboratory competence as follows.
· Repeatability (r) and Intra-laboratory Reproducibility (R)
· Limit of determination
· Estimates of Uncertainty
· Trend plots of PT data
· Sensitivity and Specificity
· IQC reference strains
Session 7
External quality control for microbiology laboratories
Dr. Susan Passmore then discussed the Proficiency Test (PT) scheme in term of ISO 17025 requirements below.
·
·
·
· 4.9 – Failures investigated as non-conforming work
The relevant standard related to PT including ISO 17043:2010, ISO TS 22117:2010, ISO 13528:2005 and ISO 7218:2007 (section 15.3); ILAG Guide 13:2007, EA-4/18:2010, EA-4/10:2002 (Section 12.2), ILAC-P9 and EA-2/10 as well as, IUPAC Harmonized Protocol for PT.
Then a video from Heath Protection Agency (HPA) was demonstrated.
The following flow showed the External Quality Assessment (EQA) through participate PT scheme.
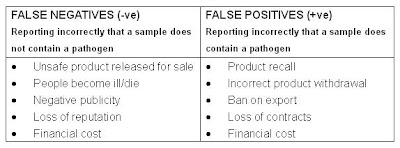
Impact of incorrect results
The HPA EQA samples were showed.
Food Examination Performance Assessment Scheme (FEPAS) cycle were demonstrated.
The following diagrams showed the Z-scores and J-scores used in PT and scheme distribution plot for each participated laboratories competence.
Session 8
Internal quality control including microbiological media QC
The last session of day 2 was internal QC including Media QC. The ISO 17025 requirements related IQC were:
·
·
·
·
The relevant ISO and other documents were briefed such as ISO 7218:2007 (Section 15.1), ISO 14461-1/-2:2005 and EA-4/10:2002 (Section 12.1).
Moreover, the ISO 17025 requirements related media QC were included.
·
·
·
The relevant ISO and other documents for media preparation and performance were ISO 11133-1/-2 and EA-4/10:2002 (Section 7).
The following diagrams showed the solid media QC in Productivity Ratio (PRs) with suspensions of target organism(s).
The suitable and unsuitable inoculums were demonstrated.
Control charts for PR were showed.
Dr. Susan Passmore introduced how to check some ready-prepared media through physical tests including appearance, colour and opacity or clarity established by visual examination, biochemical and other identification test, as well as, performance test.
Reference:
Session 7
EPTIS database listing all available PT schemes for all area at http://www.eptis.bam.de/en/index.htm
Heath Protection Agency: http://www.hpa.org.uk/
Session 8
WFCC WDCM strain numbers – http://www.wfcc.nig.ac.jp/WDCM_Reference_Strain_Catalogue.pdf



























沒有留言:
發佈留言